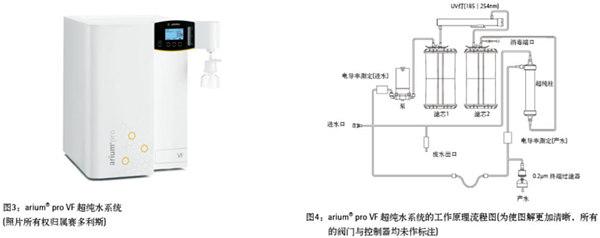
Development of Flow Cytometry Flow cytometry was initially developed for medical applications but has since evolved into a versatile tool used across various fields, including cell counting, classification, and biomarker detection [7]. Although the first study on using flow cytometry for plant cell nuclear analysis was published in 1973 [8], plant DNA analysis with this technique did not gain significant momentum until the late 1980s. Since then, researchers have increasingly adopted flow cytometry in plant research. Botanists primarily use it to measure the DNA content within plant cell nuclei. For a detailed explanation of its principles and applications, refer to Reference 9. Figure 2a generally illustrates how a flow cytometer suspends dyed or targeted particles (ranging in size from 0.2 μm to 150 μm [7]) in a stream of hydrodynamically focused liquid through an electronic detection system. In plant studies, nuclei are first stained with a fluorescent dye such as DAPI (4',6-diamidino-2-phenylindole) or propidium iodide. Each suspended nucleus is then exposed to a light beam, typically a laser or UV light. The scattered light is detected, and the fluorescence intensity is measured, which corresponds to the DNA content of each particle. By analyzing thousands of intact nuclei, researchers can determine the DNA content of different tissues, as seen in the peaks shown in Figure 1. The quality and purity of the solution used for sample preparation and analysis are crucial, as the physical and chemical properties of thousands of particles are analyzed simultaneously every second. If low-quality or unsuitable water is used, contaminants may fluoresce and create "noise," interfering with results and leading to inaccurate assessments. Therefore, high-purity ultrapure water is essential for reliable flow cytometry experiments. Ultrapure water produced by an ultrapure water system meets ASTM Class I standards. An ultrapure water system capable of producing ASTM Class I ultrapure water is provided by Sartorius (Göttingen, Germany). Using ArUPH2O, produced by the arium® pro VF water system, the authors evaluated the impact of ultrapure water on analytical results by examining the reproductive pathways of angiosperms. This was done by comparing results obtained using ArUPH2O and standard sheath solutions (0.04% sodium azide, 0.01% detergent) from Partec GmbH. The arium® pro VF system (Figure 3) generates ultrapure water from pretreated drinking water by removing all contaminants. Continuous circulation and a stable flow rate are maintained via a pressure-controlled pump system. Conductivity is monitored at both the inlet and outlet ports, or at the production port. The arium® pro VF system described in this article (which shares the same technical design as the current model, as shown in Figure 3) uses two different filter cartridges. These contain special activated carbon and mixed bed ion exchange resin to produce ultrapure water with very low total organic carbon (TOC) levels. Additionally, the system includes an integrated UV lamp that emits at 185nm and 254nm for oxidizing organics and killing bacteria. The system also features an integrated ultrafiltration module acting as a tangential flow filter. This membrane traps colloids, microorganisms, endotoxins, RNA, and DNA. A 0.2μm end filter is installed at the outlet to remove particulates and bacteria after ultrapure water is produced. Refer to Figure 4 for the complete purification process. Materials and Methods Seed samples were collected from hexaploid ranunculus under free pollination conditions. Seeds were crushed in a plastic petri dish with 300 μl of extraction buffer (CyStain UV Precise P, Partec GmbH), incubated for 10 minutes at room temperature, and then processed into a 5-mL plastic tube with 30 μl of cell suspension (CellTric®, Partec GmbH). After adding 1.2 mL of staining buffer (CyStain UV Precise P), the sample was analyzed using the blue fluorescent channel of a flow cytometer (CyFlow Space, Partec GmbH; see Fig. 2b). Results A total of 61 individual seeds were analyzed to reconstruct their reproductive pathways. According to the standard procedure, 30 seeds were suspended in sheath fluid, while 31 were suspended in ArUPH2O (conductivity: 0.055 μS/cm or 18.2 MΩ·cm resistance at 25°C). On average, 2329 nuclei were tested per sample, representing 73% of the total counted particles, with the remaining 27% attributed to background or G2 phase signals. For all seeds, the average peak position of the embryo and endosperm was calculated based on the mean of each peak's enrichment. Discussion In summary, the test results showed minimal differences, confirming that the arium system is an excellent, fast, and cost-effective alternative for ploidy analysis in plant materials. High-quality arium ultrapure water has proven effective in achieving reproducible results in lost cell seed screening techniques. However, additives like antibiotics and detergents can prevent biofilm formation and maintain humidity in containers, pipes, and flow chambers, potentially affecting long-term or short-term system performance. Suppliers often recommend adding these substances to homemade sheath fluids. In conclusion, ArUPH2O is readily available for flow cytometry in plant cells. As lost cell technology becomes more important in applications such as tumor cell detection, cell quantification, morphological differentiation, cell cycle analysis, DNA-RNA content estimation, and apoptosis detection, ArUPH2O offers new opportunities in emerging technologies utilizing flow cytometry. View full story References 1. Johri, BM Embryology of Angiosperms. Springer-Verlag: Berlin, Germany, 1984. 2. Nogler, GA Gametophytic Apomixis. In: Embryology of Angiosperms; Johri, BM, Ed. Springer-Verlag: Berlin, Germany; pp 475–518. 3. Battaglia, E. The male and female gametophytes of angiosperms—an interpretation. Phytomorphology 1951, 1, 87–116. 4. Asker, SE; Jerling, L. Apomixis in Plants. CRC Press: Boca Raton, FL, 1992. 5. Hojsgaard, DH; MartÃnez, EJ et al. Competition between meiotic and apomictic pathways during ovule and seed development results in clonality. New Phytologist 2013, 197, 336–47 (and supporting information in the online version of this article). 6. Dittrich, W.; G?hde, W. Patent DE 1815352, Flow-through Chamber for Photometers to Measure and Count Particles in a Dispersion Medium; 1968. 7. en.wikipedia.org/wiki/Flow_cytometry. 8. Heller, FO DNS-Bestimmung an Keimwurzeln von Vicia faba L. mit Hilfe der Impulscytophotometrie. (DNA estimation on radicles of Vicia faba L. using pulse cytophotometry; translation of the original German title by Dr. Herbig.) Berichte der Deutschen Botanischen Gesellschaft 1973, 86, 437–41. 9. Dolezel, J. Flow cytometric analysis of nuclear DNA content in higher plants. PhyTOChem. Anal. 1991, 2, 143–54. 10. Matzk, F.; Meister, A. et al. An efficient screen for the reproductive pathways using mature seeds of monocots and dicots. Plant J. 2000, 21, 97–108. 11. Paun, O.; H?randl, E. Evolution of hypervariable microsatellites in apomictic polyploid lineages of Ranunculus carpaticola: directional bias at dinucleotide loci. Genetics 2006, 174, 387–98. Linte remover,Best Seller Lint Removers,Best Seller Lint Removers,Clothing Lint Removers Zhejiang Hisun Electrical Appliance Co.,Ltd , https://www.cn-hisun.com