Figure 1: Measuring particle mass in a fluid Figure 2: Configuration of a Resonance Mass Spectrometer (Malvern Archimedes System) Figure 3: (from left to right) (a), (b), and (c): Aggregate detection using the Malvern Archimedes system Figure 4: Demonstration of the ability to distinguish between protein aggregates and contaminated silicone oil droplets Portable Storage Bag,Lightweight Storage Box,Moisture-proof Storage Bag,Space-saving Storage Bag,Lunch box Dongguan Jinying Handbags Co.,Ltd , https://www.jinyingbag.com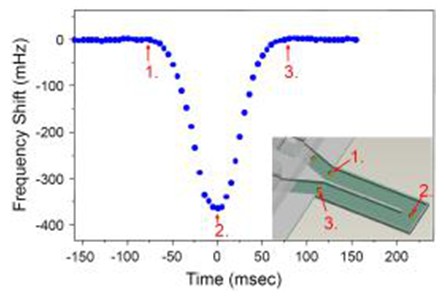

Application of Resonance Quality Test Method in Biopharmaceutical Development
**Contributor:** Malvern Instruments
**Title:** Application of Resonance Mass Measurement in Biopharmaceutical Development
**Author:** Dr. Lisa Newey-Keane, Biopharmaceutical Product Manager at Malvern Instruments
**Company Number:** MAL/JOB/2720
**Word Count:** 2286
**Image:** 4 in total (available only at the end of the file; high-resolution files available separately)
**Figure 1:** Measuring particle mass in a fluid
**Figure 2:** Configuration of the resonance mass test instrument (Archimedes system, Malvern Instruments)
**Figure 3:** (from left to right) (a), (b), and (c): Aggregate detection using the Malvern Archimedes system
**Figure 4:** Demonstration of the ability to distinguish between protein aggregates and contaminated silicone oil droplets
---
**Application of Resonance Mass Measurement in Biopharmaceutical Development**
**Author:** Dr. Lisa Newey-Keane, Biopharmaceutical Product Manager, Malvern Instruments
In the rapidly evolving biopharmaceutical industry, predicting and measuring protein aggregation remains one of the most significant challenges in drug formulation. Dr. Lisa Newey-Keane, a biopharmaceutical product manager at Malvern Instruments, introduces a novel analytical method that enhances the study of protein aggregation, offering greater accuracy and insight into the stability and quality of biological drugs.
With the increasing investment in biomolecular research, analytical testing has become a focal point in the biopharmaceutical sector. These developments are not only expensive but also subject to stringent regulatory oversight, making it essential to develop more advanced and reliable measurement techniques. Industry experts often highlight that analytical bottlenecks can significantly hinder drug development, creating a pressing need for better tools and methods.
Unlike small molecule drugs, which are typically synthetic and crystalline, protein-based formulations are inherently non-uniform and complex. This complexity makes assessing their purity and efficacy far more challenging. Protein solutions may contain various impurities such as aggregates, misfolded proteins, or fully denatured molecules. As a result, the analytical techniques used to ensure quality and provide data for pre-formulation and formulation differ significantly from those used for traditional pharmaceuticals. This complexity poses new challenges for both manufacturers and regulators.
Selecting the right candidate molecules involves extensive physicochemical testing to identify those that might become problematic during downstream processing. One critical question is how these molecules behave in the final formulation. Protein aggregation, in particular, is a major concern due to its potential to trigger immune responses. Regulatory bodies have raised concerns about protein aggregates ranging from 0.2 to 2 microns in size, yet current particle sizing methods lack the precision to quantify them effectively. The Resonance Mass Measurement (RMM) technique, implemented in the Malvern Archimedes system, addresses this gap by enabling accurate particle counting across a wide size range—from 50 nm to 5 microns.
---
**Technical Overview**
Resonance Mass Measurement (RMM) relies on a mechanical resonance structure that detects changes in mass. When particles pass through the system, they alter the resonant frequency of the structure, allowing for precise mass measurements based on frequency shifts. The Malvern Archimedes system uses microfluidic channels integrated with MEMS (Micro Electro Mechanical Systems) sensors. These sensors feature tiny cantilevers that resonate at specific frequencies, changing as the sample flows through the microfluidic channel.
The frequency shift is measured using a laser focused on the cantilever, with the signal sent to a photodiode detector. Each particle passing through the sensor causes a measurable change in frequency, enabling the calculation of mass, particle size (equivalent sphere), and surface area. Additionally, the system can determine sample concentration, density, volume, and polydispersity.
---
**Quantitative Analysis of Protein Aggregates**
Protein aggregates typically start at the dimer level and can grow to tens of microns in diameter. While flow microscopy is often used for larger particles, RMM extends the measurement range down to sub-micron sizes, filling a critical gap in analysis. This is especially important for detecting aggregates that may pose immunogenic risks and are of growing regulatory concern.
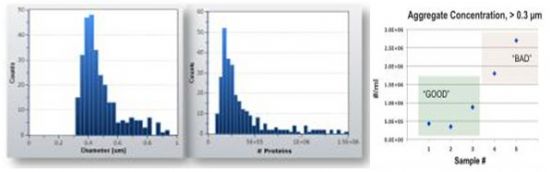
Figure 3(a) shows the particle size distribution of submicron IgG protein aggregates in 4 μL of formulation buffer. Using RMM, the concentration of aggregates larger than 300 nm was measured at 4 × 10ⶠper mL. The mass-based approach allows the particle size distribution to be expressed in terms of the number of protein molecules forming each aggregate (Figure 3(b)). After applying shear stress, Figure 3(c) demonstrates an increase in aggregate concentration from 300 nm to 1 μm, indicating a potential cascade of aggregation and highlighting poor formulation quality under stress.
---
**Detection of Contaminants**
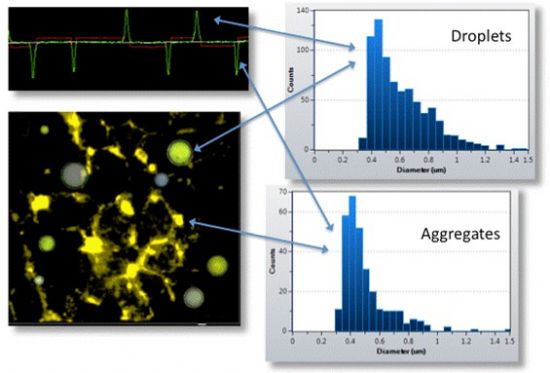
Another key challenge in protein formulation is the presence of contaminants, such as silicone oil droplets used as lubricants in syringes and containers. These droplets can resemble protein aggregates in size, leading to false positives in some measurement methods. While silicone oil is generally considered safe, its interference can affect the accuracy of results.
RMM distinguishes between protein aggregates and silicone oil droplets by measuring buoyancy. For example, in Figure 4, protein aggregates—denser than the suspension buffer—appear as negative peaks in the frequency trajectory. In contrast, silicone oil droplets, which are less dense, cause positive peaks due to their lower mass effect on the sensor. This distinction ensures more accurate and reliable data.
---
**Current Applications and Benefits**
The success of RMM technology lies in its ability to address critical particle size ranges in protein aggregation studies. It also offers practical advantages, such as requiring only 100 μL of sample with minimal preparation, even for viscous materials. This makes it ideal for early-stage biologics development, where small sample volumes and high costs are common.
By enabling the detection and quantification of particles in the 50 nm to 5 μm range, RMM provides valuable insights into formulation stability and performance. Its ability to measure buoyancy, dry mass, and particle size makes it a powerful tool for characterizing proteins and ensuring product quality.
At present, RMM is being used in the early stages of biopharmaceutical development to detect and quantify aggregates, supporting the principle that "the earlier the discovery, the lower the cost." As the industry continues to evolve, RMM stands out as a reliable and innovative solution for improving analytical capabilities in biopharmaceutical research.
---
**End of Text**
[Image]