coffee table set,marble tea table,Tea Table,stone tea table Kumusi (Dongguan) Furniture Co., Ltd. , https://www.coombesfurniture.com
Use of gene guns in aseptic workstations
**Equipment and Materials**
1. GDS-80 gene gun with a 4.5 mm barrel, pressure regulator, and hose (WGB09O001) (Wealtec)
2. Aseptic laminar flow hood
3. 3 cm target spacer (Wealtec)
4. High-purity helium gas (99.999%)
5. Sample: Zea mays L. embryos
**Experimental Procedure**
1. Before starting the experiment, adjust the output pressure of the GDS-80 gene gun according to the manufacturer’s instructions to ensure even particle distribution. After setting the parameters, clean the sample cannula and barrel with 70% ethanol to prevent contamination.
2. Prior to use, thoroughly clean and disinfect the aseptic table. It is recommended to expose it to UV light for at least 30 minutes to kill any residual microorganisms.
3. Sterilize all equipment, including the barrel, sample cannula, target spacer, and forceps, before placing them inside the aseptic table.
4. Wipe the exterior of the GDS-80 gene gun body and hose assembly with 70% ethanol before placing them on the aseptic table.
5. Assemble the loading tube and sample tube, ensuring the gene gun is properly placed within the aseptic table and connected to the system.
6. Set the pressure regulator to 50 psi and check that the gas flow rate is approximately 10–15 L/min.
7. Prepare the DNA plasmid/gold powder solution in advance. The ratio should be 0.5 µg DNA per 0.148 mg gold particles.
8. Use a 3 cm or 6 cm target spacer during the bombardment process. After the procedure, transfer the sample to MS medium for further incubation.
9. Incubate the sample for at least two days, then stain it with GUS staining solution for one day.
10. Observe the results under a microscope to assess successful gene transformation.
**Results**
(a)
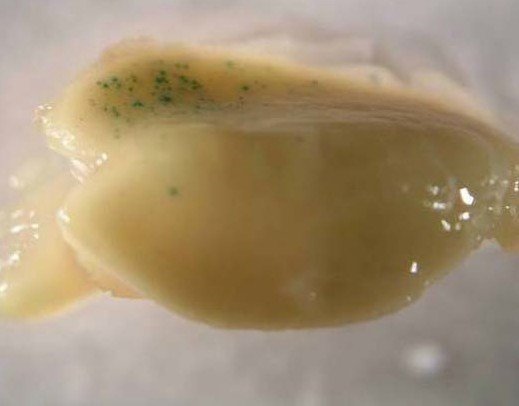
(b)

**Figure 1. Introduction of the GUS gene into corn embryos using the GDS-80 gene gun at 50 psi with a 3 cm target spacer.**
(a) Normal conditions without contamination.
(b) Contaminated media.
**Discussion**
Maintaining sterility is critical when working in an aseptic environment. Even minor lapses can lead to severe contamination, which may interfere with the detection of the GUS protein. If the culture dish becomes contaminated, even a pure DNA sample may fail to show positive results due to low expression levels.
Contamination often occurs due to several common factors, and careful attention can help avoid these issues:
a. **Contamination from the gene gun equipment**:
During operation, it's easy to overlook the need to fully sterilize the barrel and sample loading sleeve. Simply spraying 70% ethanol on the outside is not sufficient. Microbes can remain on the inner surfaces, leading to cross-contamination. To prevent this, all parts should be either autoclaved at 121°C for 30 minutes or soaked in 70% ethanol for over 20 minutes, then dried and placed in the aseptic table.
b. **Contamination from the plant sample itself**:
Plant samples collected from the field are often exposed to various microbes. Proper surface sterilization is essential. A common method involves soaking the sample in a bleach-Tween-20 solution for more than 20 minutes, followed by thorough rinsing with sterile water. The sample should then be dried on a sterile petri dish before being used in the experiment.
c. **Unskilled handling in the aseptic cabinet**:
Many users neglect important steps while working in the aseptic table. Always wash hands thoroughly and disinfect them with 70% ethanol before entering. Once inside, avoid removing hands from the cabinet unless necessary. When moving items in or out, make sure to disinfect hands again before returning. Avoid opening culture dishes too much and never let hands or objects pass over the open lid. Use only sterilized pipette tips and replace them after each use. Handle samples with sterilized forceps, and never touch anything other than the sample. If a sample falls, do not attempt to recover it—discard it.
d. **Poor maintenance of the aseptic table**:
Regularly check and replace the filters in the aseptic table as needed. Over time, their effectiveness decreases, increasing the risk of contamination. Even with proper maintenance, it's good practice to wipe down the internal work area with 70% ethanol before each use.
In summary, strict sterilization protocols must be followed throughout the entire experimental process. While different labs may have slightly different SOPs, the goal remains the same: to prevent contamination. Following these guidelines carefully will greatly improve the success of your experiments.